
Research focus at Marianski group
The research interest at Marianski lab at Hunter College CUNY ranges from a single bond formation under applied mechanical force to noncovalent interactions between the most complex biomolecules in biology. The tools we apply to investigate these problems are tailored to the specific demands of these problems. For instance, investigating a bond formation process necessitates methods that explicitly consider the molecular electronic structure, such as Density Functional Theory (DFT). On the other end of the spectrum, understanding recognition between large biomolecules, such as binding of synthetic carbohydrate receptors to a glycan portion of a protein, merits methods of classical molecular simulations. Finally, some other problems, such as glycoside reactions in an enzymatic center under mechanical stress, will require both quantum and classical approaches merged in a QM/MM scheme.
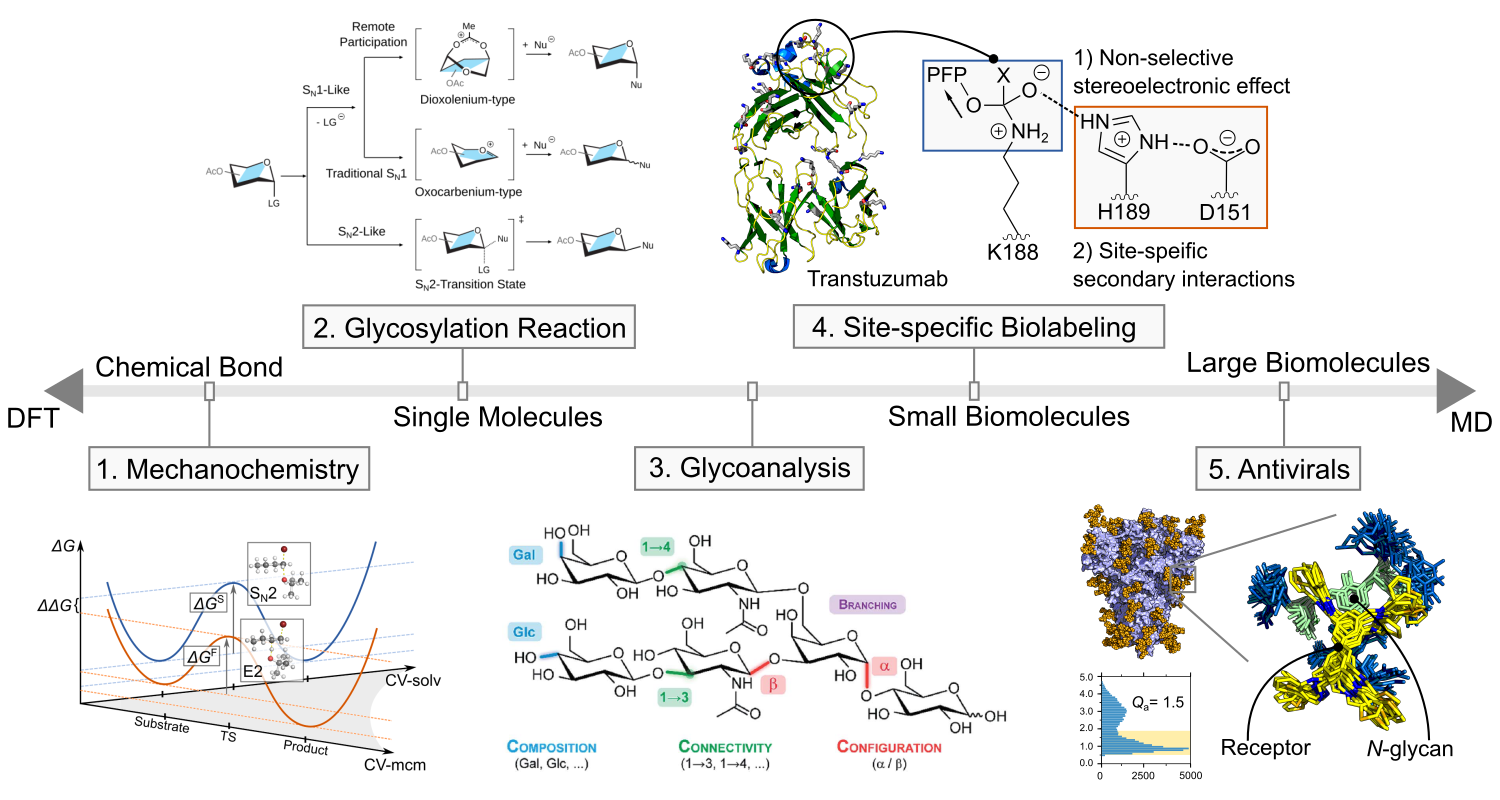
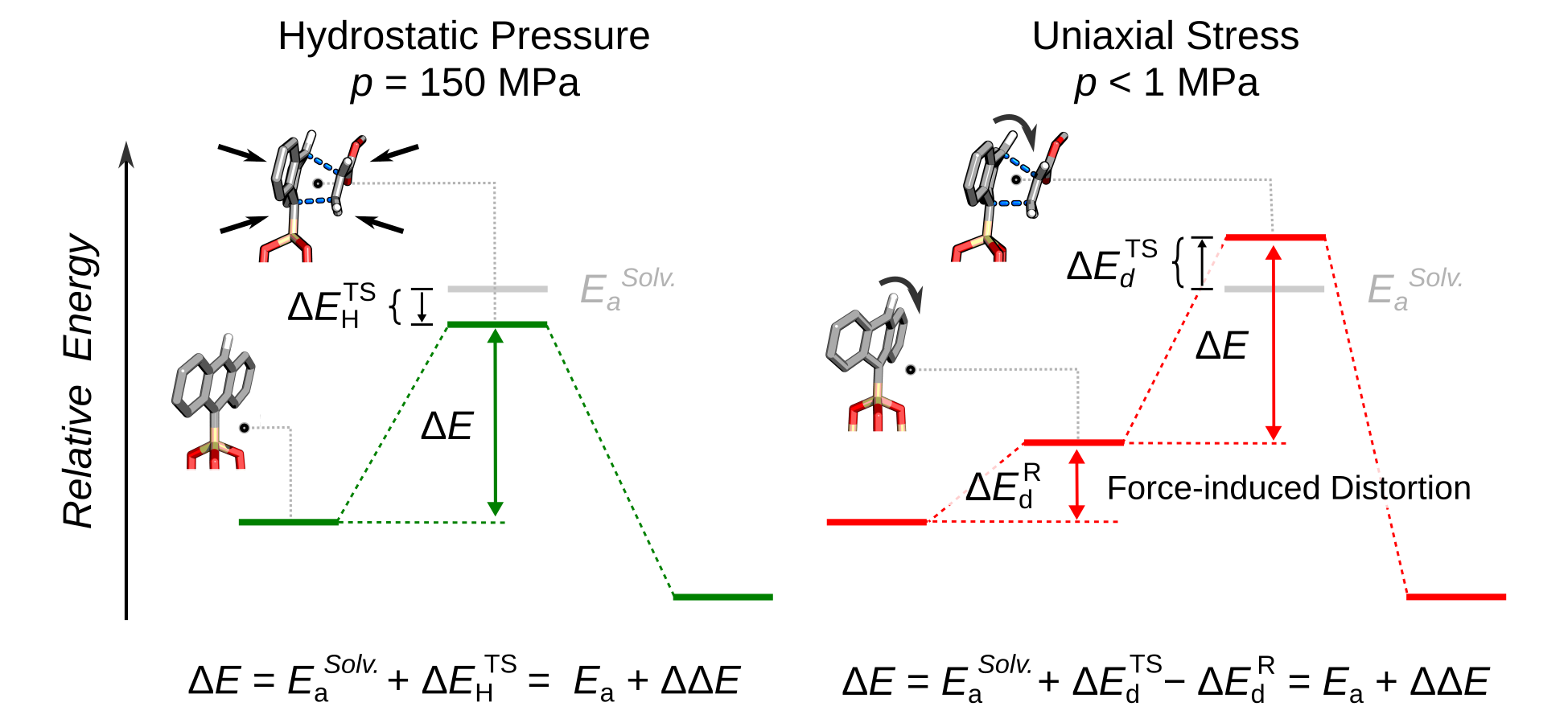
1. Mechanochemistry: Altering organic reactions with mechanical forces
Background: The organic solvents employed in the great majority of organic reactions carried out in industry account for >60% of all chemical manufacturing waste. Because of the energy demand of so-called solvothermal syntheses, where solvent and heat drive reactions, the chemical industry consumes 37% of the total energy used in manufacturing. Replacing solvothermal with mechanically-activated organic chemistry – where force, rather than heat and solvent, drives the making and breaking of covalent bonds – can solve the waste and energy challenges of organic synthesis. Mechanochemically-activated conditions can also yield stereoisomers and regioisomers that are not favored under solvothermal conditions. Despite its many potential advantages, significant gaps in the understanding of reaction kinetics under mechanochemical activation limits adoption of mechanochemistry in chemical synthesis and manufacturing.
This project is a part of a new NSF CCI: Center for the Mechanical Control of Chemistry.
Key papers:
[2] '’Kinetics of primary mechanochemical covalent-bond-forming reactions’‘ Y. Zholdassov, R. W. Krow, M. A. Shlain, M. Patel, M. Marianski, A. B. Brauschweig RSC Mechanochemistry 2024, 1, 11-32 doi: 10.1039/D3MR00018D
[1] '’Acceleration of Diels-Alder Reactions by Mechanical Distortion’‘ Y. S. Zholdassov, L. Yuan, S. R. Garcia, R. W. Kwok, A. Boscoboinik, D. J. Valles, M. Marianski, A. Martini, R. W. Carpick, A. B. Braunschweig Science, 2023, 380, p.~1053 doi: 10.1126/science.adf52
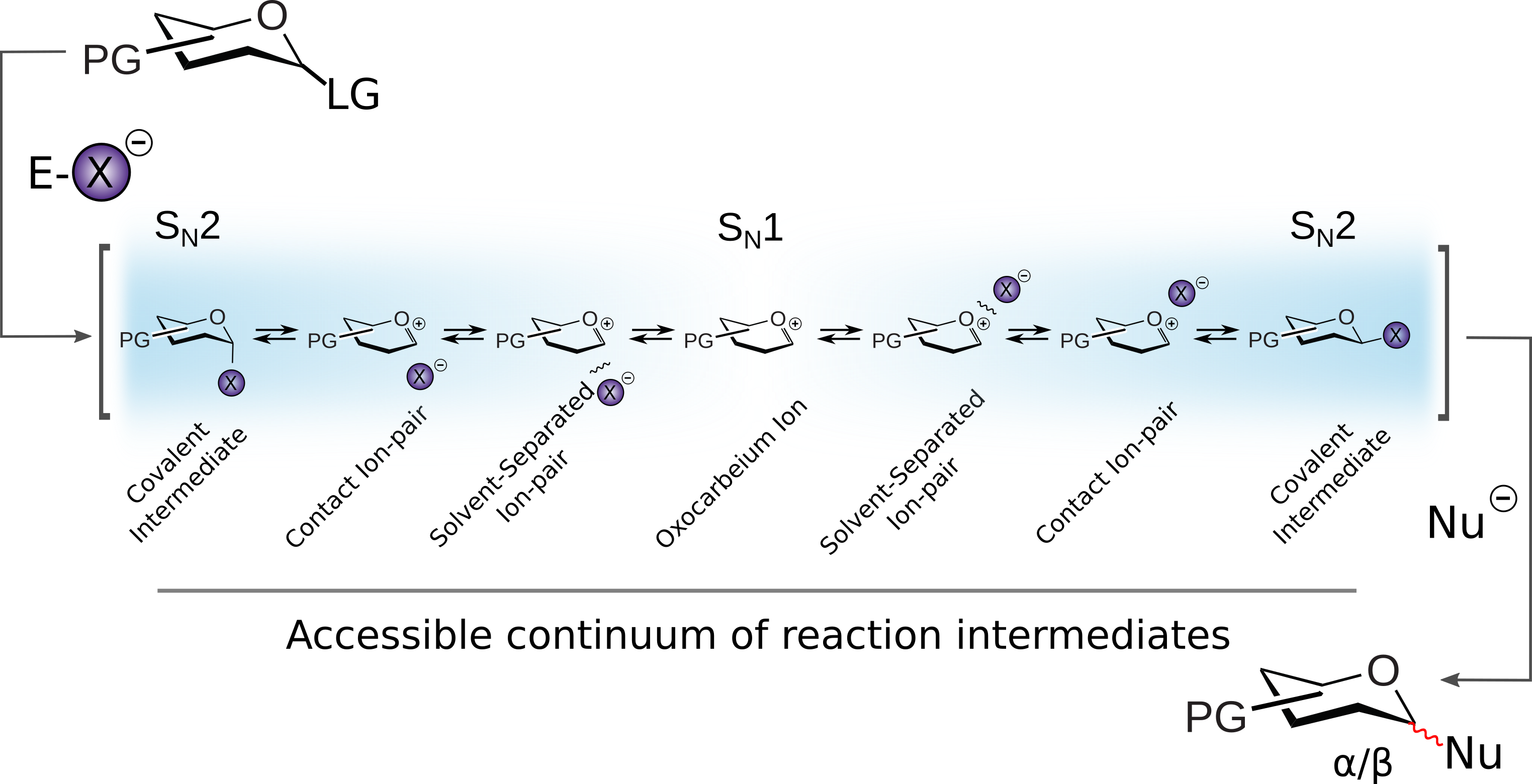
2. Glycosylation Reaction: Reviving the reaction mechanism with molecular simulations
Carbohydrates - or glycans - play an essential role in a variety of biochemical processes, such as cell-cell communication, and immune system recognition. Being the identification card of a cell, glycans have given rise to over 170 commercial drugs and vaccines that exploit their functions. However, carbohydrate-based drugs and vaccines remain niche on account of their complex and time-consuming synthesis, which necessitates scrupulous control over the regio- and stereoselctivity of the glycosidic bond formation.
The glycosylation reaction couples a glycosyl donor to an acceptor and proceeds through an idiosyncratic glycosyl-donor intermediate, which is believed to exist as a continuum of potential intermediates. The dominant structure will then orchestrate the reaction mechanism, and the ability to in fluence this dominant intermediate is essential to establish better stereochemical control over the reaction. In previous work, by combining high-throughput DFT calculations, and a cryogenic-ion IR spectroscopy (prof. Kevin Pagel/FHI of the Max Planck Society, Berlin), we have shown that the glycosyl donors intermediates equipped with the protecting groups at positions C2[1] or C4[2] adopt a dioxolenium-type of a structure.
Importantly, we demonstrated that the formation of this type of an intermediate correlates with the stereoselectivity of the glycosylation reaction, which proofs that the fine-tuning of the stereoelectronic properties of the protecting groups can significantly influence the outcome of the reaction.
Key papers:
[4] '’Evaluating Participation Modes in Peracetylated Glycosyl Cations’‘ N. Geue, K. Greis, S. Omoregbee-Leichnitz, C. Kirschbaum, G. Meijer, G. von Helden, P. H. Seeberger, M. Marianski, K. Pagel, Organic Letters, 2026, In Print doi:10.1021/acs.orglett.5c04756
[3] '’Stereoelectronic Effect of Protecting Groups on the Stability of Galactosyl Donor Intermediates’‘ R. W. Kwok, R. Rutkoski, P. Nagorny, M. Marianski, Molecules, 2025, 30(2), 218 doi:10.3390/molecules30020218
[2] '’Direct Evidence for Remote Participation in Galactose Building Blocks during Glycosylation Revealed by Cryogenic Vibrational Spectroscopy’‘ M. Marianski, E. Mucha, K. Greis, D. S. Moon, A. Pardo, C. Kirschbaum, D. A. Thomas, G. Meijer, G. von Helden, K. Gillmore, P. Seeberger, K. Pagel, Angewandte Chemie International Edition, 2020, 59, pp.~6166-6171 doi: 10.1002/anie.201916245
[1] '’Unravelling the structure of glycosyl cations via cold-ion infrared spectroscopy’‘ E. Mucha*, M. Marianski*, F. Xu, D. A. Thomas, G. Meijer, G. von Helden, P. H. Seeberger, K. Pagel, Nature Communications, 2018, 9, p.~4174 doi: 10.1038/s41467-018-06764-3
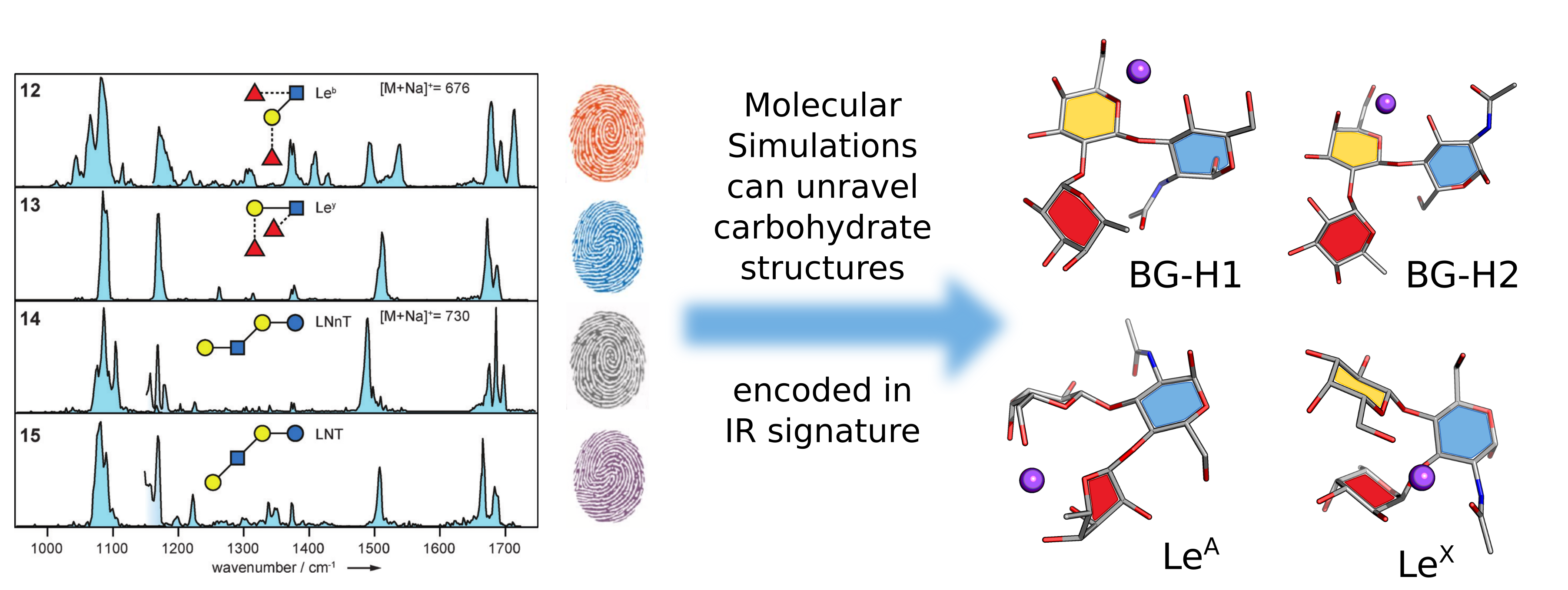
3. Glycoanalysis: Fingerprints please – fragment-based analysis of glycans in a gas phase
Glycan expression and composition in the glycocalyx – the layer of glycolipids, glycoproteins, and glycopolymers on the surface of all eukaryotic cells as well as many bacteria and viruses – have been correlated to developmental status, cell type, and the disease state of a cell. These cell-, tissue-, and species-specific inventories of glycans comprise the “sugar code”, but much of this encoded information remains undeciphered, leaving critical gaps in knowledge about biological processes and networks that could be exploited to treat disease. Thus, there remains a need for new tools to ana lyze and utilize the information buried within the glycocalyx. One promising technique is combining gas-phase analysis of carbohydrates, using techniques such as mass-spectrometry (MS), ion-mobility spectrometry (IMS) or action IR spectroscopy, with high-throughput DFT calculations.
While working at the FHI Berlin, we have established that 1) DFT provides excellent theoretical framework to study carbohydrates in a gas phase;[1] and 2) glycans have unique IR fingerprints that can be used to decode their structure.[2] Synergy between these two techniques enabled study of the sequence-structure relationship in glycans,[3] and glycosphingolipids.[4] We proposed, that the combination of computational and experimental techniques can be used to create a reference library to facilitate fragment-based analysis of glycans.[5] In a recent contribution, we have combined several gas-phase techniques and high-throughput DFT calculations to reveal the rearrangement products of the fucose migration in blood group epitopes.[6] The computational work has also catalyzed the development of Carbohydrate Parser in python (CarPpy) which streamlines the DFT computations, and the subsequent analysis, of carbohydrates.[See Developed Code]
Key papers:
[8] '’Mechanistic study on the sulfate migration in glycosaminoglycans during MS fragmentation’‘ L. Polewski, M. Yaman, M. Tokić, M. Marianski, K. Pagel, Communications Chemistry, 2026, In Print doi:10.1038/s42004-026-01939-2
[7] '’Reinvestigation of the internal glycan rearrangement of Lewis a and blood group type H1 epitopes’‘ V. Kontodimas, M. Yaman, K. Greis, M. Lettow, K. Pagel, M. Marianski, Physical Chemistry Chemical Physics, 2024, 26, 14160-14170 doi:10.1032/D3CP04491B
[6] '’Decoding the Fucose Migration Product during Mass-Spectrometric Analysis of Blood Group Epitopes’‘ M. Lettow, K. Greis, E. Mucha, T. R. Lambeth, M. Yaman, V. Kontodimas, C. Manz, W. Hoffmann, G. Meijer, R. R. Julian, G. von Helden, M. Marianski, K. Pagel Angewandte Chemie International Edition, 2023, e202302883 doi: 10.1002/ange.202302883
[5] '’In-depth structural analysis of glycans in the gas phase’‘ E. Mucha*, A. Stuckmann*, M. Marianski*, W. Struwe, G. Meijer, K. Pagel, Chemical Science, 2019, 10, pp.~1272-1284 doi: 10.1039/C8SC05426F
[4] '’Unravelling the Structural Complexity of Glycolipids with Cryogenic Infrared Spectroscopy’‘ C. Kirschbaum, K. Greis, E. Mucha, L. Kain, S. Deng, A. Zappe, S. Gewinner, W. Schöllkopf, G. von Helden, G. Meijer, P. B. Savage, M. Marianski, L. Teyton, K. Pagel, Nature Communications , 2021, 12, p. 1201 doi: 10.1038/s41467-021-21480-1
[3] '’Fucose Migration in Intact Protonated Glycan Ions: A Universal Phenomenon in Mass Spectrometry’‘ E. Mucha, M. Lettow, M. Marianski, D. A. Thomas, W. B. Struwe, D. J. Harvey, K. Pagel, Angewandte Chemie International Edition, 2018, 57, pp.~7440–7443 doi: 10.1002/anie.2018.01418
[2] '’Glycan fingerprinting using cold-ion infrared spectroscopy’‘ E. Mucha, A. I. Gonz'{a}lez Fl'{o}res, M. Marianski, D. A. Thomas, W. Hoffmann, W. B. Struwe, H. S. Hahm, S. Gewinner, W. Sch"{o}llkopf, P. H. Seeberger, G. von Helden, K. Pagel, Angewandte Chemie International Edition, 2017, 56, pp.~11248-11251, doi: 10.1002/anie.201702896
[1] '’Assessing the accuracy of across-the scale methods for predicting carbohydrate conformational energies on the example of glucose and $\alpha$-maltose’‘ M. Marianski, A. Supady, T. Ingram, M. Schneider and C. Baldauf, Journal of Chemical Theory and Computations, 2016, 12, pp.~6157-6168 doi: 10.1021/acs.jctc.6b00876
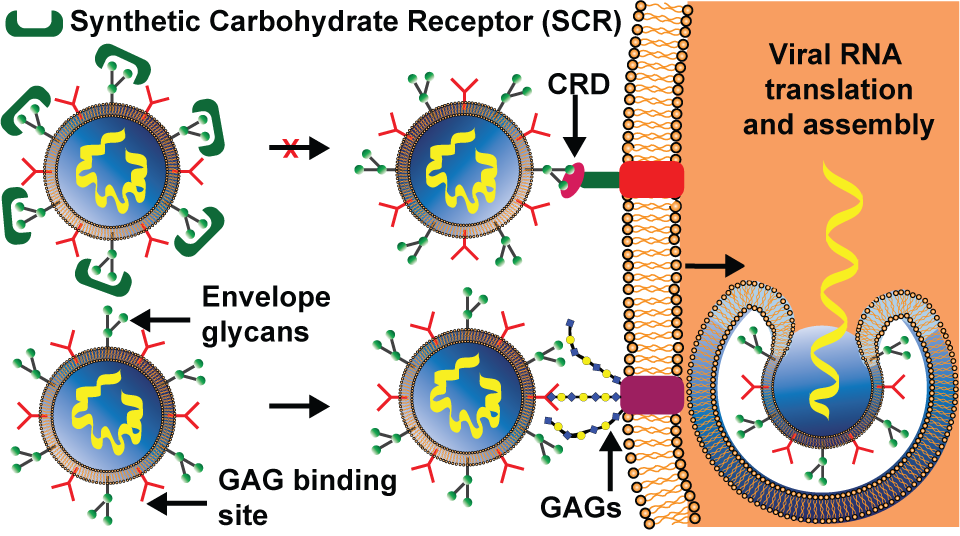
4. Antivirals: Can viral glycans be targeted to detect and stop viral diseases?
The recognition events between glycans and receptors have a central role in the viral life cycle. Understanding how these binding processes are involved in pathogen infection and reproduction could lead to new and desperately needed antiviral agents to counter the threat of viral outbreaks, especially those posed by enveloped viruses (EnV). The glycans can be either on proteins on the viral envelope, such as oligomannosides on the surface of Dengue Virus binding to the host cell protein DC-SIGN, or on the host cell, such as N-linked glycans terminated by sialic acid which are targets for influenza virus. Being able to inhibit these interactions will open new therapeutic strategies for novel antivirals.
In collaboration with prof. Adam Braunschweig (ASRC CUNY), we have synthesized a large library of over 80 synthetic carbohydrate receptors (SCRs), and characterized their binding towards biologically-relevant carbohydrates. We have established design rules to anticipate the SCR selectivity and to optimize its binding affinity.[1−4] Importantly, these SCRs show strong antiviral activity. We hypothesize that the SCRs interact with N-glycans on the surface of EnV, which leads to the inhibition of the docking of a virus to a host cell. We have demonstrated, using MD simulations, that SCRs are selective towards specific N-glycans, abundance of which correlates with the measured antiviral activity of SCRs.[5]
Key papers:
[6] '’Broad-spectrum synthetic carbohydrate receptors (SCRs) inhibit viral entry across multiple virus families’‘ S. Ezzatpour, K. Thakur, K. E. Ndede, D. W. Buchholz, A. Choi, B. Imbiakha, J. Carter, D. Onofrei, B. Eaton, E. Postnikova, M. Murphy, B. C. Tapia, D. Bello, S. Pasari, A. Russo, M. Babayev, G. P. Holland, M. R. Holbrook, S. L. Caddy, S. J. Moran, S. M. Davachi, I. A. Monreal, J. Sahler, V. Ortega, J. M. Miranda, G. R. Whittaker, M. C. Jager, S. K. Bhagwat, P. Chopra, G. Jan-Boons, M. Marianski, A. B. Braunschweig, H. C. Aguilar Science Advances, 2025, 11, eady3554 doi:10.1126/sciadv.ady3554
[5] '’Binding of Synthetic Carbohydrate Receptors to Enveloped Virus Glycans: Insights From Molecular Dynamics Simulations’‘ B. Tapia, G. Yagudayeva, M. F. Bravo, K. Thakur, A. B. Braunschweig, M. Marianski, Carbohydrate Research , 2022, 518, pp. 108574 doi: 10.1016/j.carres.2022.108574
[4] '’Regiochemical Effects on the Carbohydrate Binding and Selectivity of Flexible Synthetic Carbohydrate Receptors with Indole and Quinoline Heterocyclic Groups’‘ K. Thakur, M. A. Shlain, M. Marianski, A. B. Braunschweig, European Journal of Organic Chemistry , 2021, 37, pp. 5262-5274 doi: 10.1002/ejoc.202100763
[3] '’Flexible Synthetic Carbohydrate Receptors as Inhibitors of Viral Attachment’‘ F. Bravo, M. Lema, M. Marianski, A. B. Braunschweig, Biochemistry , 2021, 60, pp. 999-1018 doi: 10.1021/acs.biochem.0c00732
[2] '’Synthesis and Binding of Mannose-Specific Synthetic Carbohydrate Receptors’‘ F. Bravo, K. Palanichamy, M. A. Shlain, F. Schiro, Y. Naeem, M. Marianski, A. B. Braunschweig, Chemistry - European Journal , 2020, 26, pp. 11782-11795 doi: 10.1002/chem.202000481
[1] '’Binding Studies on a Library of Induced-Fit Synthetic Carbohydrate Receptors with Mannoside Selectivity’‘ K. Palanichamy, F. Bravo, M. A. Shlain, F. Schiro, Y. Naeem, M. Marianski, A. B. Braunschweig, Chemistry European Journal, 2018, 24, pp.~13971–13982 doi: 10.1002/chem.201803317